COMPANY PROFILE
About Biogeny Diagnostics
Biogeny Diagnostics Pvt. Ltd. is an ISO 13485:2016 and ZED Gold certified company with its plant and office located in Navsari, Gujarat. Established in 2009, Biogeny began its operations as a manufacturer of hematology reagents. Over the years, it has evolved into a leading instrumentation company, offering a diverse range of instruments that are now recognized as a leading brand in the market. To support its field activities, Biogeny has a strong team of more than 80 field personnel dedicated to marketing. Additionally, the company provides exceptional after-sales support with a dedicated team of over 100 service engineers across India, ensuring comprehensive service coverage.
Biogeny has expanded its global presence by collaborating with leading international companies to offer a comprehensive product range to its customers. The company is committed to employing highly qualified and talented individuals, adopting new technologies for product development, and continuously improving product quality and services to meet the highest standards.
"To bring industry best products within reach of the domestic market
True Guiding Light of the Diagnostic Industry"
Explore Our Product Range
Export
Beacon has a large portfolio of its global customers in SAARC, South East Asia, Middle East, Africa and Europe. Beacon is expanding its coverage with a dedicated team working for Exports. For export inquiries you can contact to us at exports@beaconindia.com .
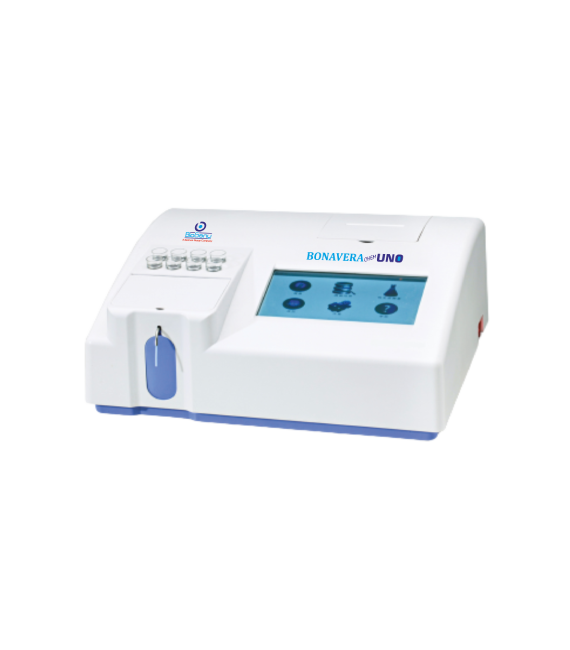
Biochemistry
Serology
Hematology Reagents
Turbidimetry
Rapid Tests
What our Clients
are saying
Reason to choose us
Choose Us for Unmatched Excellence: Our commitment to innovation and affordability ensures top-tier solutions. With exceptional service and a focus on building lasting relationships, we're your trusted partner in diagnostics
Call to ask any question